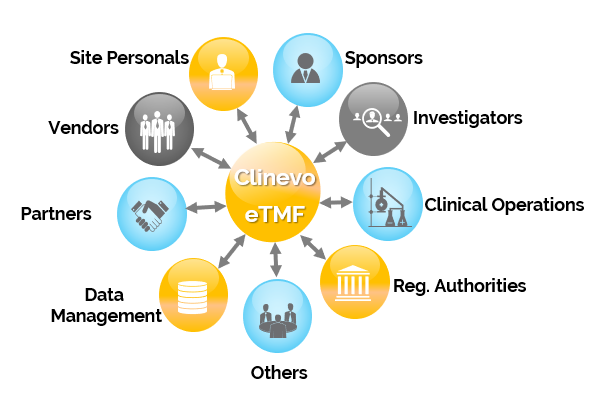
Clinevo electronic Trial Master File (eTMF) is an easy to use electronic Trial Master File in electronic (digital content) format for organizing and storing documents, images, and other digital content of clinical trials.
Clinevo electronic Trial Master File (eTMF) has inbuilt DIA reference model and meets regulatory guidelines including:
 Inbuilt & Configurable DIA TMF Structure
Inbuilt & Configurable DIA TMF Structure  File Planning & Milestones Setup
File Planning & Milestones Setup Document Lifecycle and Version Control
Document Lifecycle and Version Control Advanced Analytics
Advanced Analytics Bulk / Quick upload with Dynamic Indexing
Bulk / Quick upload with Dynamic Indexing External Systems Integration
External Systems Integration Sharing and Collaboration
Sharing and Collaboration  Faster User Adoption
Faster User Adoption Anytime, Anywhere & Any device
Anytime, Anywhere & Any device Regulatory Inspection Ready
Regulatory Inspection Ready Extendable Document Management System
Extendable Document Management SystemDo you want to Know more Enquire Now
Call us now:
USA : +1 - 315 232 8028
INDIA : +91 - 44 6645 5983
Reach us Now:
info@clinevotech.com