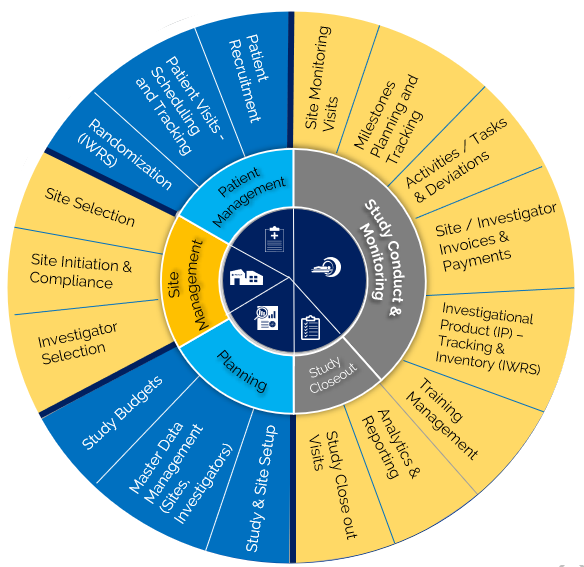
Clinevo Clinical Trial Management System (CTMS) is a cloud based, highly configurable, "end-to-end platform which" helps manage all aspects of clinical trials including :
Clinevo Clinical Trial Management System (CTMS) meets the regulatory guidelines including security, access control, change controls, audit trails, and the best system validation.
A Clinical Trial Management System (CTMS) is a software application that supports the Clinical trial processes. It provides infrastructure, consisting of three components: Clinical Data Management System (CDMS), Site Management System (SMS), and a Patient Recruitment Database or a Clinical Trial Web Portal that enable a site to plan, implement, monitor, analyze and report on clinical research activities to maximize the probability of delivering high-quality data within time and budget constraints.
 One Clinical Trial System
One Clinical Trial System Compliance Alerts
Compliance Alerts Dynamic workflows
Dynamic workflows Integrated Master Data Management
Integrated Master Data Management Feature-rich and flexible
Feature-rich and flexible Regulatory Inspection Ready
Regulatory Inspection Ready Real time monitoring and reporting
Real time monitoring and reporting Cost Effective & High Performing
Cost Effective & High Performing Faster User Adoption
Faster User Adoption Anytime, Anywhere & Any device
Anytime, Anywhere & Any deviceDo you want to Know more Enquire Now
Call us now:
USA : +1 - 315 232 8028
INDIA : +91 - 44 6645 5983
Reach us Now:
info@clinevotech.com