Remote Monitoring | rSDV
Clinevo Clinical Trial Remote Monitoring Systems(RMS) is a web based & easy-to-use software which enables companies to perform remote Source Data Verification (rSDV) / remote Source Data Review (rSDR) without having monitors travelling to the sites.
Clinevo Remote Monitoring System for Clinical trials - meets regulatory guidelines including :
Redacting personal / sensitive information
Version control
Security and access control
Change controls
Digital content archiving
Audit trails, and System validation.

Remote Clinical Monitoring is a web-based and easy-to-use software that enables sites and CROs to perform remote SDV/RSDV easily and securely.
It was developed by Clinevo – a leading enterprise GRC SaaS company with extensive analytics domain expertise. This solution allows clients to monitor data from all types of clinical trials such as multi-center, multi-country, Phase II-IV (in any geography), or single-center (if required). Our clientele comprises large Biotechs (>100), Pharma (>300), and CROs (~350). We have successfully handled FDA inspections for multiple prestigious
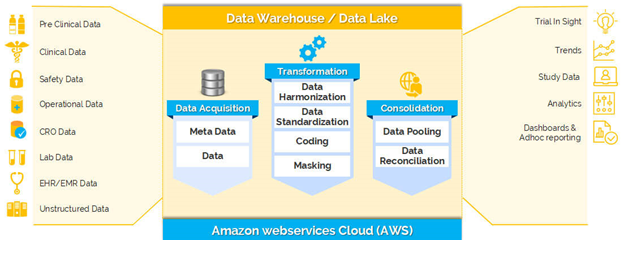
Below picture depicts how Clinevo Clinical Trial Remote monitoring system (RMS) addresses remote Source data verification (rSDV) process in Clinical Trials
Comprehensive Pharmacovigilance Solution
Clinevo Pharmacovigilance Software is a powerful, AI-driven drug safety database designed to streamline case processing, ensure compliance, and provide advanced analytics for pharmaceutical companies, CROs, and regulatory teams.
Live Tracking & Statuses
Configurable Source Data Verification
File Planning & Milestones Setup
Document Lifecycle and Version Control
Efficiency & Cost Optimization
Clinevo Safety is built on a simplified technology stack, ensuring cost-effectiveness, easy maintenance, and faster user adoption.
Advanced Analytics
Clinevo Remote Monitoring System for Clinical Trials (RMS) provides advanced analytics to track Completeness, Quality, Timeliness and Source Data Verification (SDV) compliance of a clinical trial.
Bulk / Quick upload
Clinevo Clinical Trial Remote Monitoring software allows users to upload hundreds of documents in one go using a simple drag and drop & provides dynamic indexing options for documents
Redaction of Information
System provides options to redact sensitive information from the site / patient / visit documents.
Smart Insights & Accessibility
Clinevo Pharmacovigilance Software offers intelligent analytics and real-time compliance alerts while ensuring seamless access from anywhere.