10 Essential Features of a Modern eTMF
July 20, 2020 | Uncategorized | No Comments

Electronic Trial Master File (eTMF) solutions are quickly becoming an essential option for managing clinical trial documentation. This helps in replacing paper trial master files and clunky file shares. Earlier, organizations used to face a lot of challenges that come from managing trial master file content on paper or in file share systems. The effort required to locate, manage and collaborate on important documents in a timely manner has caused many organizations to search for more efficient solutions
eTMF systems are gaining widespread acceptance, however, many organizations that seek this solution are still not completely familiar with the core features of an eTMF. In the following article, you will get to know some of the most powerful features found in an eTMF software that clinical organizations should be looking for.
Below are the features you should essentially look for while searching the perfect eTMF system for your organization:
1. Prebuilt DIA TMF Reference Model & Configurable Organization / Sponsor Specific TMF Reference models:
The TMF Reference Model provides standardized taxonomy and metadata and outlines a reference definition of TMF content using standard nomenclature. DIA is the reference model for TMF which is widely used and accepted by most of the global companies and regulators. The eTMF system should come with a prebuilt DIA reference model hierarchy and the system should automatically create the structures for Study, Country, and Site level documents.
Although the DIA reference model is important, it is equally important that the system should allow organizations to define their own TMF structures and sponsor specific organization folders. The system should support customizing the existing DIA TMF model or configuring Organization / Sponsor specific TMF structures.
2. File Planning & Milestones Setup for Study, Countries and Sites:
The system should allow companies to setup milestones at the study level, country level, and site level and plan on what documents and how many numbers of mandatory and optional documents that are targeted to be collected.
Once a file plan and milestones plan are setup for a study, the system should be capable of tracking the clinical trial document in accordance to the plan and alert users if some of the sites are not meeting the plan and milestone due dates
This is one of the mandatory features for an eTMF system. Without this feature the system becomes a simple File sharing platform.
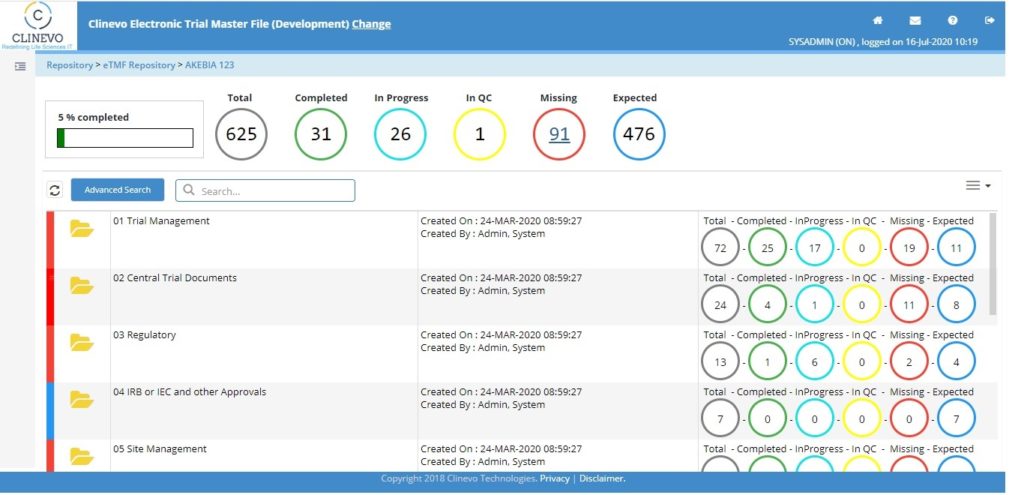
3. Live Tracking of Missing Documents & Document Statues:
Based on the file plan setup for a Study, Country and Site, the system should highlight the status of the document along with missing documents to monitor the TMF compliance at every level.
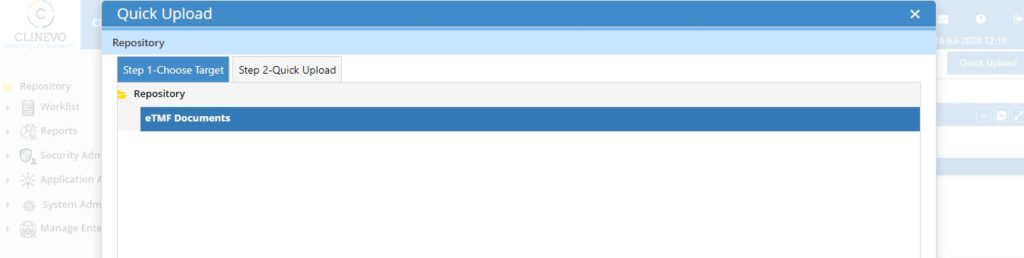
4. Bulk Upload / Quick Upload & Flexible File Indexing:
The system should allow users to upload documents in bulk where in hundreds of documents can be dragged and drop in one go. This feature will be very useful when there is a remote site that can not place the documents in the right TMF hierarchy. Such remote sites can upload all the documents into a TEMP directory into the system and later the central coordinator/CTA or CRA can index the documents to the right TMF structure.
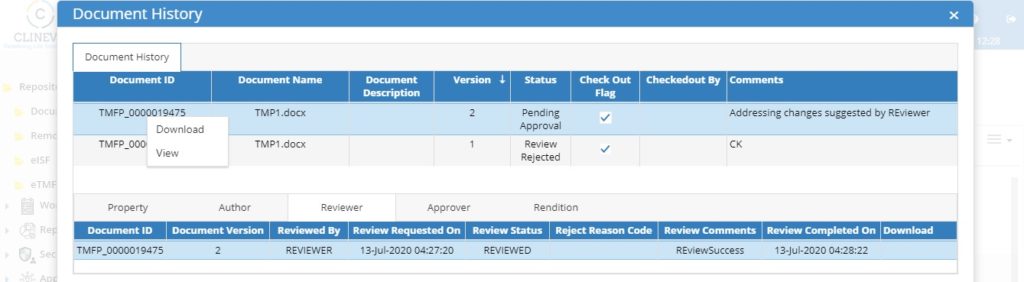
5. Automated Version Controlling:
The system should be capable of tracking each version of the document along with its description of changes every time a document is modified/ re-uploaded.
The system should be capable of having automated versioning based on the user edits and at the same time system should ensure only one user can modify the document at a given point in time to make sure every version of the document contains who has done what changes.
6. Electronic Signatures & Converting to PDF:
The system should be capable of capturing the electronic signatures of the authors, reviewers and approvers and print the same when it’s downloaded as PDF with inbuilt PDF conversion options. System should also be capable of printing configurable watermarks and the change descriptions along with electronic signatures.
If you want to have a 100% electronic TMF, then the signature technology also needs to meet the requirements of 21 CFR Part 11 electronic signatures when signing records.
7. External System Integration
The system should be able to integrate with CDMS/ CTMS systems to get the study, site, patient and other information and should be able to dynamically create Directory Structures and upload documents
8. Remote monitoring with Sharing and Collaboration
A good eTMF system should allow a user to invite external parties to upload and work collaboratively on a document system in a controlled method as it is essential for such systems to integrate and exchange information with one another.
9. Inspection Ready & Regulatory Compliance
The system should have Inventory and complete audit reports which can be shared with the Regulatory auditors during the audit which shall give complete overview and history on how the study documents are captured and filing in the eTMF repository.
The system should also maintain an audit trail for every operation and should have fully compliant eSignatures for all stakeholders.
It should also comply with all current and regulations including 21 CFR Part 11, ANNEX 11, GxP, and GDPR.
10. Advanced Analytics to track Completeness, Quality & Timeliness
The system should consist of interactive dashboards having analytics which will help business users to gain powerful and actionable insights. Importantly, the system should have dashboards to monitor the completeness of the study, Quality of the documents collected, and timeliness of the Study / Country / Site document filing and reviews.
