Clinevo Technologies & Galaxi Consulting partners to reduce 75% IT cost in Pharmacovigilance, Clinical Data Warehousing and Automation Solutions in the Europe market

Galaxi Consulting is a leading Life Sciences and Manufacturing consulting firm focused on finding the best solutions for Pharma and Biotech clients across Europe and the UK, currently working with the Fortune 100 Pharmaceutical and Healthcare companies. They are also digital recruiters specialized in sourcing and deploying the topmost applicants in the Life Sciences, Biotech, Pharma and Manufacturing sector.

Clinevo Technologies is a Software Development Company specialized in developing and implementing robust technology solutions for Life Sciences R&D. They help Pharma, Biotech and CROs in reducing their time and cost in Clinical trials by implementing innovative technologies that involve Data Warehousing, Analytics, Collaboration, Automation, and Artificial Intelligence.
Clinevo Technologies currently has 3 products on a GxP Cloud:

Clinevo Safety: Clinevo Safety is a cloud based, easy to use, regulatory Compliant, AI enabled, end-to-end Pharmacovigilance / Drug Safety system. This all-in-one system provides PV Intake, Case processing, AI, Analytics, Submissions/AS2 gateway and Safety signals capabilities under one platform.
Clinevo DW and Automation: Clinevo Data Warehousing and Business Process Automation Console is a secured, regulatory compliant Clinical Trials Data Warehouse to Acquire, Store, Transform, Consolidate and Report diverse data from clinical trials in one place and AUTOMATE any of the manual, cumbersome business processes. This platform can enable companies to perform Cross Study Analysis, Data mining, Predictive Analytics, etc.
Clinevo eTMF: Clinevo eTMF is an extendable electronic trial master file in electronic (digital content) format for organizing and storing documents, images, and other digital content of clinical trials.
The Partnership:
Clinevo and Galaxi Consulting are pleased to announce a partnership that extends the power of Clinevo’s innovative cloud-based technology solutions and Galaxi Consulting’s international talent pool of Life Sciences specialists and aimed at helping Pharmaceutical, Biotech and CROs with high performing, GxP compliant Pharmacovigilance and Clinical trial solutions.
This partnership is primarily aimed at cutting down the IT cost in the Life Sciences R&D domain by 75% with unique cloud-based IT solutions.
Arunkumar Devaraj, Director of Life Sciences at Clinevo, said, “We have developed a lot of unique features that would make our products as the best when compared to other existing products in the market. We already have a lot of highly satisfied customers from USA and India market and we are excited to be partnering with Galaxi Consulting and introducing our products to its customers in Europe and the UK”.
Gaurav Sharma, Director, Galaxi Consulting said, “We are highly impressed with the Clinevo’s end-to-end product strategy which brings in a lot of benefits with huge cost savings to the customers who are into Pharmacovigilance and Clinical Trials. With our vast consulting experience in Europe and the UK, we are looking forward to delivering high-quality solutions to the customers”.
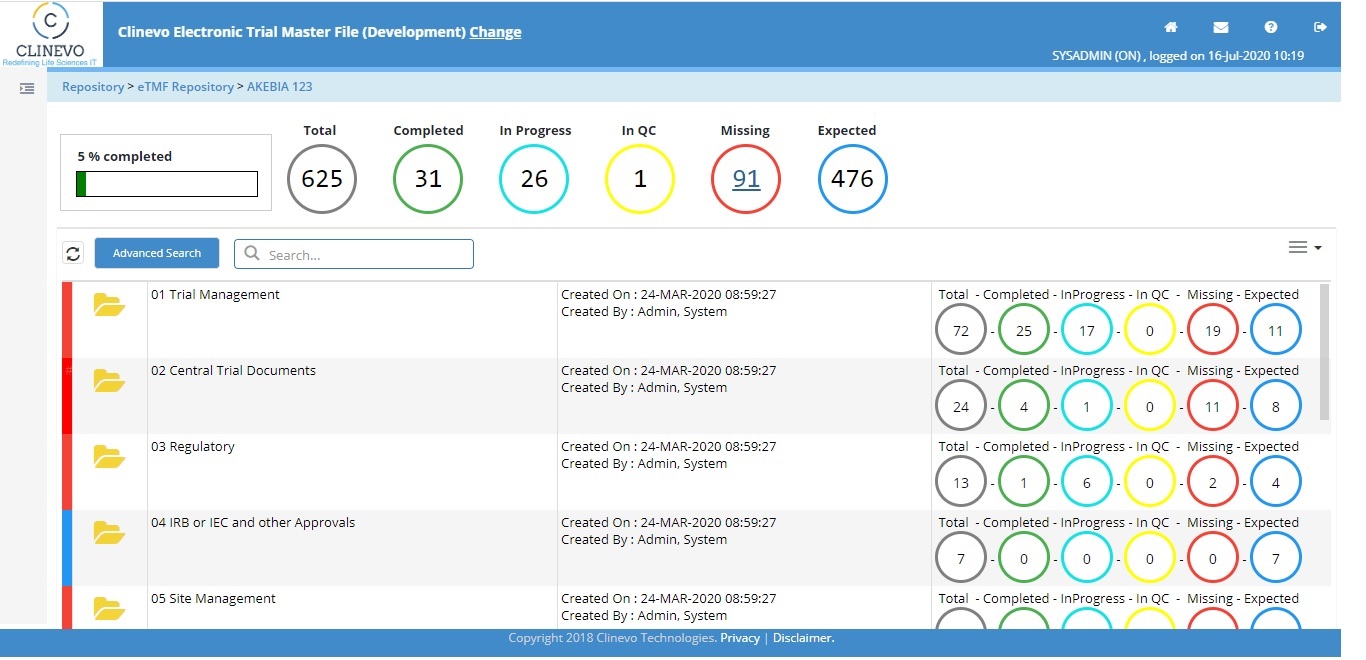
3. Live Tracking of Missing Documents & Document Statues:
Based on the file plan setup for a Study, Country and Site, the system should highlight the status of the document along with missing documents to monitor the TMF compliance at every level.
4. Bulk Upload / Quick Upload & Flexible File Indexing:
The system should allow users to upload documents in bulk where in hundreds of documents can be dragged and drop in one go. This feature will be very useful when there is a remote site that can not place the documents in the right TMF hierarchy. Such remote sites can upload all the documents into a TEMP directory into the system and later the central coordinator/CTA or CRA can index the documents to the right TMF structure.

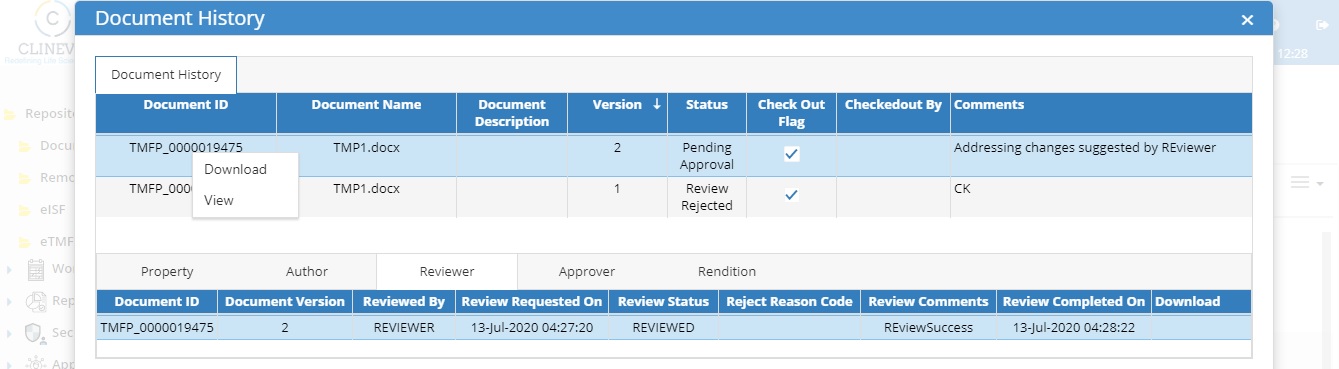
5. Automated Version Controlling:
The system should be capable of tracking each version of the document along with its description of changes every time a document is modified/ re-uploaded.
The system should be capable of having automated versioning based on the user edits and at the same time system should ensure only one user can modify the document at a given point in time to make sure every version of the document contains who has done what changes.
6. Electronic Signatures & Converting to PDF:
The system should be capable of capturing the electronic signatures of the authors, reviewers and approvers and print the same when it’s downloaded as PDF with inbuilt PDF conversion options. System should also be capable of printing configurable watermarks and the change descriptions along with electronic signatures.
If you want to have a 100% electronic TMF, then the signature technology also needs to meet the requirements of 21 CFR Part 11 electronic signatures when signing records.
7. External System Integration
The system should be able to integrate with CDMS/ CTMS systems to get the study, site, patient and other information and should be able to dynamically create Directory Structures and upload documents
8. Remote monitoring with Sharing and Collaboration
A good eTMF system should allow a user to invite external parties to upload and work collaboratively on a document system in a controlled method as it is essential for such systems to integrate and exchange information with one another.
9. Inspection Ready & Regulatory Compliance
The system should have Inventory and complete audit reports which can be shared with the Regulatory auditors during the audit which shall give complete overview and history on how the study documents are captured and filing in the eTMF repository.
The system should also maintain an audit trail for every operation and should have fully compliant eSignatures for all stakeholders.
It should also comply with all current and regulations including 21 CFR Part 11, ANNEX 11, GxP, and GDPR.
10. Advanced Analytics to track Completeness, Quality & Timeliness
The system should consist of interactive dashboards having analytics which will help business users to gain powerful and actionable insights. Importantly, the system should have dashboards to monitor the completeness of the study, Quality of the documents collected, and timeliness of the Study / Country / Site document filing and reviews.




